Palladium
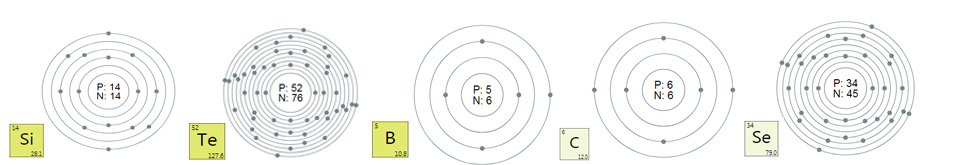
Palladium is a rare mineral, a noble metal of the
platinum group, silvery in color, does not tarnish in air. Discovered by
the English chemist and mineralogist W.H. Wollaston, who discovered
palladium in native platinum in 1803, malleable and malleable. More
fusible compared to platinum, easily rolled and drawn into wire. Melting
point 1552 ° C. Paramagnetic. Let's dissolve in HNO3, in hot
concentrated Н2SO4 and aqua regia. Palladium has an extremely high
affinity for hydrogen, in the form of a powder it is able to absorb a
volume of hydrogen 900 times the volume of the metal itself. Compared to
other platinum metals, it is less resistant to oxidants.
Palladium is plastic, microadditives of nickel, cobalt, rhodium or
ruthenium improve the mechanical properties of Pd, increase the
hardness.
Insoluble in water; density - 12.02 (20 ° C, g / cm^3); under special
conditions forms colloidal palladium and palladium black. Of all the
metals of the platinum group, palladium is the lowest melting point. The
melting point is 1554 ° C (in some sources 1552 ° C); boiling point
approx. 2940 ° C. Heat of fusion - 37.8 cal / g; specific heat at 20 ° C
- 0.0586 cal / (g o deg); electrical resistivity at 25 ° C - 9.96 μOhm /
cm; thermal conductivity - 0.161 cal / (cm o sec o deg). Paramagnet,
that is, magnetized in an external magnetic field in the direction of
this field.
In its pure form, palladium has a beautiful silvery-white color. As with
all precious metals, its color does not change over time.
Pure palladium is a fairly soft metal. Its hardness is 373 MPa Brinell,
which roughly corresponds to the hardness of platinum (392 MPa) and
exceeds the hardness of gold and silver (245 MPa). The hardness of pure
palladium increases with cold forging or rolling. When annealed, the
hardness decreases again. Pure palladium cannot be used in jewelry; it
will be extremely sensitive to mechanical stress. However, adding small
amounts of other metals to palladium, especially nickel or ruthenium,
greatly increases its hardness. For example, for the production of
jewelry in Europe and North America, palladium 950 is used, i.e. the
jewelry contains 95% pure palladium. The remaining 5% is usually
ruthenium or copper. For the manufacture of jewelry, palladium alloys
with silver and nickel of 500 or 850 tests, an alloy with 850 tests of
copper are used. The wear resistance of palladium jewelry is
approximately equal to that of platinum and is higher than that of gold
and silver jewelry.
Palladium is often used as a catalyst, mainly in the hydrogenation of
fats and the cracking of petroleum. Palladium chloride is used as a
catalyst and to detect trace amounts of carbon monoxide in air or gas
mixtures.
Palladium chloride is used in electroplating as an activating agent in
the galvanic metallization of dielectrics - in particular, the
deposition of copper on the surface of laminated plastics in the
production of printed circuit boards in electronics.
Palladium and palladium alloys are used in electronics for
sulfide-resistant coatings (an advantage over silver).
In particular, palladium is constantly used for the production of
high-precision precision resistance reochords (military and aerospace
engineering), including in the form of an alloy with tungsten (for
example, PDV-20M). The use in these units is due to the high wear
resistance of palladium, which is ideal for its use in contact groups.
By the way, palladium wire reochords were widely used in civilian
equipment, and palladium in its pure form was used in the contacts of
step switches of control and recorder machines, in the contacts and
strings of the ISS (multiple coordinate connectors) of ATCK (automatic
telephone exchange coordinate stations) produced with 1982 to 1987 USSR.
Palladium is also a part of ceramic capacitors (type KM), with high
temperature stability of the capacitance in high-frequency radio
broadcasting, radio communication, and television equipment.
In alloys used in jewelry (for example, to obtain a gold-palladium alloy
- the so-called "white gold"). Palladium even in a small concentration
in the alloy (about 1%) changes the color of the gold-based alloy from
yellow to silvery-white. The main alloys of palladium with silver used
in jewelry have a fineness of 500 and 850 for silver (since they are the
most technologically advanced when machined and decorative). Limited
edition commemorative coins are sometimes minted from palladium.
Palladium and its alloys are used to make medical instruments, parts for
pacemakers, and dentures;
In some countries, a small amount of palladium is used to obtain
cytostatic drugs - in the form of complex compounds, similar to cis-platinum.
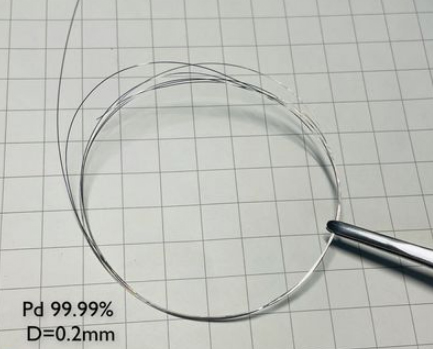

Palladium is a rare mineral, a noble metal of the
platinum group, silvery in color, does not tarnish in air. Discovered by
the English chemist and mineralogist W.H. Wollaston, who discovered
palladium in native platinum in 1803, malleable and malleable. More
fusible compared to platinum, easily rolled and drawn into wire. Melting
point 1552 ° C. Paramagnetic. Let's dissolve in HNO3, in hot
concentrated Н2SO4 and aqua regia. Palladium has an extremely high
affinity for hydrogen, in the form of a powder it is able to absorb a
volume of hydrogen 900 times the volume of the metal itself. Compared to
other platinum metals, it is less resistant to oxidants.
Palladium is plastic, microadditives of nickel, cobalt, rhodium or
ruthenium improve the mechanical properties of Pd, increase the
hardness.
Insoluble in water; density - 12.02 (20 ° C, g / cm^3); under special
conditions forms colloidal palladium and palladium black. Of all the
metals of the platinum group, palladium is the lowest melting point. The
melting point is 1554 ° C (in some sources 1552 ° C); boiling point
approx. 2940 ° C. Heat of fusion - 37.8 cal / g; specific heat at 20 ° C
- 0.0586 cal / (g o deg); electrical resistivity at 25 ° C - 9.96 μOhm /
cm; thermal conductivity - 0.161 cal / (cm o sec o deg). Paramagnet,
that is, magnetized in an external magnetic field in the direction of
this field.
In its pure form, palladium has a beautiful silvery-white color. As with
all precious metals, its color does not change over time.
Pure palladium is a fairly soft metal. Its hardness is 373 MPa Brinell,
which roughly corresponds to the hardness of platinum (392 MPa) and
exceeds the hardness of gold and silver (245 MPa). The hardness of pure
palladium increases with cold forging or rolling. When annealed, the
hardness decreases again. Pure palladium cannot be used in jewelry; it
will be extremely sensitive to mechanical stress. However, adding small
amounts of other metals to palladium, especially nickel or ruthenium,
greatly increases its hardness. For example, for the production of
jewelry in Europe and North America, palladium 950 is used, i.e. the
jewelry contains 95% pure palladium. The remaining 5% is usually
ruthenium or copper. For the manufacture of jewelry, palladium alloys
with silver and nickel of 500 or 850 tests, an alloy with 850 tests of
copper are used. The wear resistance of palladium jewelry is
approximately equal to that of platinum and is higher than that of gold
and silver jewelry.
Palladium is often used as a catalyst, mainly in the hydrogenation of
fats and the cracking of petroleum. Palladium chloride is used as a
catalyst and to detect trace amounts of carbon monoxide in air or gas
mixtures.
Palladium chloride is used in electroplating as an activating agent in
the galvanic metallization of dielectrics - in particular, the
deposition of copper on the surface of laminated plastics in the
production of printed circuit boards in electronics.
Palladium and palladium alloys are used in electronics for
sulfide-resistant coatings (an advantage over silver).
In particular, palladium is constantly used for the production of
high-precision precision resistance reochords (military and aerospace
engineering), including in the form of an alloy with tungsten (for
example, PDV-20M). The use in these units is due to the high wear
resistance of palladium, which is ideal for its use in contact groups.
By the way, palladium wire reochords were widely used in civilian
equipment, and palladium in its pure form was used in the contacts of
step switches of control and recorder machines, in the contacts and
strings of the ISS (multiple coordinate connectors) of ATCK (automatic
telephone exchange coordinate stations) produced with 1982 to 1987 USSR.
Palladium is also a part of ceramic capacitors (type KM), with high
temperature stability of the capacitance in high-frequency radio
broadcasting, radio communication, and television equipment.
In alloys used in jewelry (for example, to obtain a gold-palladium alloy
- the so-called "white gold"). Palladium even in a small concentration
in the alloy (about 1%) changes the color of the gold-based alloy from
yellow to silvery-white. The main alloys of palladium with silver used
in jewelry have a fineness of 500 and 850 for silver (since they are the
most technologically advanced when machined and decorative). Limited
edition commemorative coins are sometimes minted from palladium.
Palladium and its alloys are used to make medical instruments, parts for
pacemakers, and dentures;
In some countries, a small amount of palladium is used to obtain
cytostatic drugs - in the form of complex compounds, similar to cis-platinum.

|

|